Introduction: HCT/HGB values stipulated by WHO in 2022 1* (and 2016) are required for distinguishing pts with PV from those with either essential thrombocythemia (ET), cellular phase primary myelofibrosis (MF), or JAK2-mutated clonal hematopoiesis. We verified WHO values have 100% specificity but only approximately 75% sensitivity 2. Thus, PV pts exist with values less than WHO criteria for HCT/HGB. Distinguishing these PV pts from ET, MF or CHIP is important since prognosis and management differs. No studies to date, however, have defined the frequency and characteristics of these specific PV pts. We therefore reviewed our cohort of PV pts to identify those whose HCT and HGB values were lower than WHO. We report their clinical, hematologic, and molecular findings and their survival to date.
Methods : We queried our database of PV pts, extracting data as previously reported 3 for those with HCT/HGB values below WHO threshold at diagnosis (dx). These pts met PV diagnostic criteria by either marrow morphology, red cell mass (RCM), and/or erythropoietin (EPO) values. We recorded clinical, hematologic and JAK2 V617F variant allele frequency (VAF) findings, therapy, clinical course, MF free (MFS) and overall survival (OS).
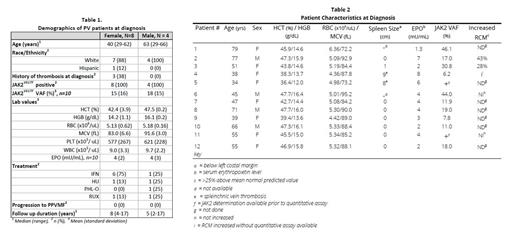
Results: Twelve of 470 (2.6%) pts had criteria of PV with HCT/HGB values below WHO. All were JAK2 V617F positive. 10 had quantitative values, with median VAF 18% (range 6.2-46.1%). Median age was 45 years. Mean erythrocyte values for males (N=4) and females (N=8) respectively were: HCT 47.5 and 42.4%, HGB 16.1 and 14.2 g/dL, RBC 5.18 and 5.13 x10 6 /uL, MCV 91.6 and 83.0 fL (Table 1). Cr-51 RCM was increased in 3. 11 pts had marrow biopsies showing varying degrees of hypercellularity and increased megakaryocytes with the characteristic morphologic changes described in PV. Patient 1 did not have Cornell review of marrow but otherwise had lab and clinical values consistent with PV (Table 2). 5 of 11 pts had an MF score of 0, 5 MF-1, and 1 MF-2. Stainable iron was absent in 5, present in 4, and not assessed in 2. Since low JAK2 VAF is associated with ET, in the 9 pts with VAF under 20%, or qualitative values only, the PV dx was made on marrow morphology and increased RCM, low EPO level, high RBC count or combinations thereof (Table 2).
Seven pts were symptomatic at dx, including fatigue, headache, and generalized abdominal discomfort. Spleen was palpable at dx in 3 pts, 1, 8, and 9 cm below the left costal margin, respectively. The last two pts presented with splanchnic vein thrombosis. Another pt had a cerebrovascular accident at dx.
Maintenance treatment was phlebotomy in 1, hydroxyurea 2, ruxolitinib 2, and rIFN 7. None of the pts progressed to MF; 1 pt died of colon cancer. Over a median follow up of 7 years, median MFS and OS have not been reached (100% MFS and 92% OS to date).
Conclusion: A small number of PV pts present with HCT/HGB below WHO threshold; however, recognition is important because of their symptoms and risk of significant thrombosis. Our 12 pts were diagnosed with PV based on a combination of either marrow morphology, EPO level, and RCM. As isotopic RCM studies are difficult to obtain, RBC and marrow morphology becomes extremely important for correct dx. The presence of marrow iron stores in 4 pts is significant since most PV pts are iron deficient at dx 4. This suggests that the HCT/HGB values observed may represent “early” disease. The HCT/HGB values in the 2 splanchnic vein thrombosis pts with significant splenomegaly represent well-known splenic sequestration and increased plasma volume, masking true polycythemia. In these cases, marrow studies and EPO values are mandatory to distinguish PV from ET. This study emphasizes the appropriate use of RCM and should prompt development of alternative methods to measure blood volume.
*2022 WHO PV diagnostic criteria 1:
Major Criteria:
1. HGB > 16.5 g/dL (men) HGB > 16.0 g/dL (women) or HCT >49% (men) HCT > 48% (women) or increased red cell mass more than 25% above mean normal predicted value
2. BM biopsy showing hypercellularity for age with trilineage growth (panmyelosis) including prominent erythroid, granulocytic and megakaryotic proliferation with pleomorphic, mature megakaryocytes (differences in size)
3. Presence of JAK2 or JAK2 exon 12 mutation
Minor criteria: subnormal serum erythropoietin level
References
1. Khoury et al. Leukemia, 2022.
2. Silver R, Krichevsky S. Haematologica, 2019.
3. Abu-Zeinah et al. Leukemia, 2021.
4. Silver R, Abu-Zeinah G. Exp Rev of Hem, 2023.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal